Question
(a)
Outline the manufacturing process for the production of steel suitable
for ships shell plating.
(b)
(i) Sketch a simple iron-carbon
equilibrium diagram and indicate a typical composition of ships steel.
(ii)
Sketch the micro-structure of this steel.
Answer.
The production of steel involves a chemical process of
melting 'pig iron' and scrap metal in a furnace. Pig iron is produced from iron ore in a
blast furnace and is 92% to 97% iron with C, Si, S, P and Mn
present as impurities.
The chemical process is to combine with and hence
remove the impurities (as gases or slag).
Most ship steel is made by a 'basic' chemical reaction e.g. lime is
added to remove P (as slag). 'Acid'
chemical reactions can also be used to produce steel from lower P composition
steels. Finally, additions are made to give desired properties to the steel.
There are three processes that may be used for
producing the steel:
Open hearth. An
open ended furnace enclosure
Electric furnace. An enclosed furnace with
an arc produced from two electrodes suspended from the roof
Oxygen process. Oxygen is injected into
the melt to refine it. Ingots of steel are now transformed into 'slabs' then
'plates' by hot rolling as shown in the following sketch.
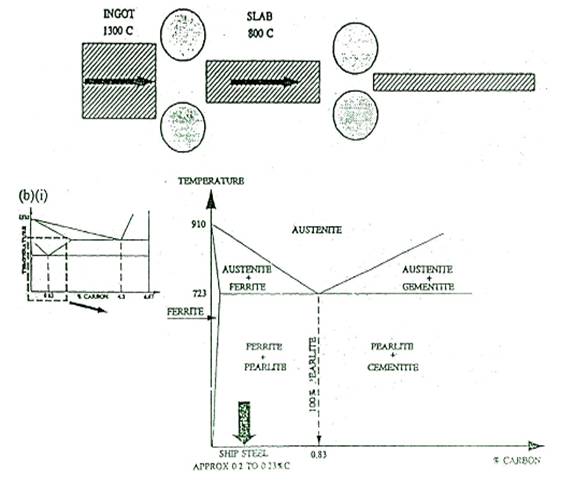
The iron carbon thermal equilibrium diagram is quite
an involved diagram as shown above.
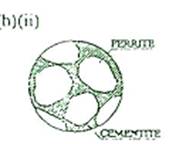
The
micro-structure of the steel would be grains of ferrite (approx 75%) within a pearlite matrix.
The pearlite is a laminated structure comprising of alternate layers of ferrite and a carbide called-cementitc (Fc3 C)