Question
(a) Surface preparation and
painting of new ship plates
(b) Design of the ships
structure and its maintenance
(c )
Cathodic protection by sacrificial anodes, of the internal
and external areas of the ship.
Answer:
(a) If the plates are
immersed in a weak solution of sulphuric acid or hyfrochloric acid
for a few hours the majority of scale is removed. This is pickling. The pickled plate must be hosed
down with fresh water on removal from the tank, allowed to dry before painting.
During drying a light coat of rust is formed and must be removed before
painting.
Flame clearing, oxy
acetylene torch having several jets is used to brush the surface, burns the
dirt and grease, loosens the surface rust,
loosens the mill scale. The surface is immediately wire brushed and
the priming coat applied while still
warm.
Mill scale is removed by
shot blasting. This removes dirt, rust and grease as well. This removes 95% to
100% mill scale and results in slightly rough surface which allows
adequate adhesion of the paint. The
plate is spray painted on emerging from the shot blasting machine.
(b) Direct connection
between dissimilar metals should be avoided or effectively insulated from each
other. Avoid trapping quantities of sea water or any electrolyte, crevices by
selection of construction members of suitable radiused
section or by sealing.
Larger
enough drains to be provided to drain water and dirt.
Tanks should be provided with drains to enable complete draining with out
leaving any residue.
The metals should be
protected prior to assembly and prevent water soaking into porous materials. Sharp edges where the paint draws
away should be removed.
If two dissimilar metals are used paint all the
surfaces or at least the more cathodic material. Weld
metal of more noble quality than the parent metal -should be used. Traces of
residual surface products (slag which is cathodic
sometimes) of welding, should be cleaned before painting.
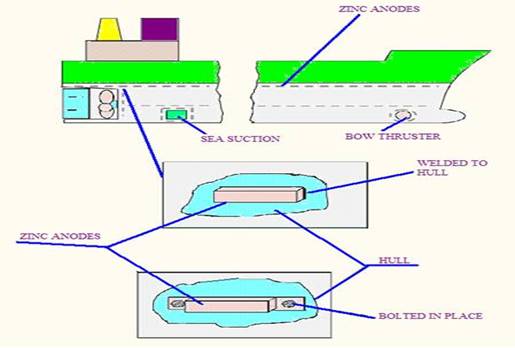
(c) Zinc or aluminium anodes are used as sacrificial anodes for the
protection of external areas of the ship. Zinc is allowed corrode in preference
to steel hull and propeller. And also protects hull due to differences in
steel. The current is provided by sacrificial anodes attached to the ships hull
which form a short circuited cell with the hull as cathode and sea water as
electrolyte. This system does not require any maintenance but a large number of
bulky anodes are required to give full protection until next drydock.
Deep water ballast tanks are
also protected by sacrificial zinc anodes. Rust is to be removed and a film of
the anode material is to be formed. This is achieved by using booster anodes
having large surface area
compared with their volume. To maintain the film main anodes having large
volume compared to surface area are fitted. These are designed to last for
three years. Protection is provide to the whole tank only if the electrolyte is in full contact, it is
necessary to press up the tank when ballasted.