REFRIGERATION
The purpose of refrigeration is to transfer heat from a cold chamber which is at a lower temperature, than that of its surroundings. Since heat will not flow freely from a body at a lower temperature to another at a higher temperature it is necessary to expend mechanical work. So a refrigeration system uses some-external source to do work on a media, called the refrigerant and follows a thermodynamic process to achieve this.
The two main refrigeration systems are absorption system and the vapour compression system.
VAPOUR COMPRESSION CYCLE :
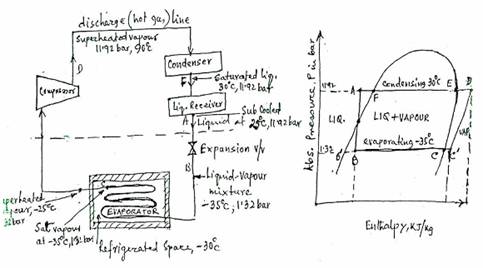
The vapour Compression cycle takes place in a closed system, comprising of a compressor, a condenser, a liquid receiver, an evaporator and a flow control or expansion valve, interconnected by discharge, liquid and suction lines.

The liquid refrigerant, e.g. R22 is stored at high pressure in the receiver. The liquid flows from the receiver through the liquid line to the expansion valve, which regulates the rate of flow to the evaporator to suit the rate of evaporation. As it passes through the expansion valve, the pressure of liquid is reduced to the evaporating pressure, so that the saturation temperature of the refrigerant entering the evaporator is below that required in the refrigerated space. As the liquid passes through the flow control valve, a portion of liquid evaporates instantly (flash gas) in order to reduce the temperature of the remaining liquid to the evaporating temperature.
The liquid vapour mixture of refrigerant then flows through the evaporator, where it extracts heat from the refrigerated space and changes to a dry saturated vapour, at
approximately the same pressure and the same temperature as that at which it left the flow control.
The evaporating pressure is maintained constant by the action of the compressor, which removes vapour from the evaporator at the same rate at which it is formed, in practice, the control system regulating the refrigerant flow is designed to ensure that the vapour leaving the evaporator is slightly superheated, thus ensuring that only dry vapour is handled by the compressor.
In the compressor, the temperature and pressure of the vapour are raised by compression. The Compressed vapour flows into the condenser, using water or air as the cooling medium. The vapour in the condenser first gives up its superheat as it is cooled from the discharge temperature to saturation temperature corresponding to condensing pressuraand then gives up its latent heat as it condenses back to a liquid. The liquid then flows from the bottom of Condenser to Receiver thus completing the cycle. When its temperature is below the condensing temperature it is said to be sub - cooled.
Line A to B - change from high to low press or expansion process (from 11.92 bar to 1.32 bar)
Line B to B' -represents the amount of liquid flashed - off in the expansion valve cooling the remaining liquid.
Line B to C - evaporation process at constant saturation temperature and pressure in the evaporator (-35°C and 1.32 bar). At point C, the refrigerant is a dry saturated vapour.
Line C to C -represents the superheat absorbed by the dry saturated vapour (from -35°C to -25°C).
Line C to D -represents the compression process in the compressor, (from 1.32 bar to 11.92 bar, -25°C to 90°C)
Line D to E - represents the superheat given off by the vapour in the condenser (from 90°C to 30°C). At point E, the refrigerant is a dry saturated vapour.
Line E to F - represents the condensation process at constant saturation temperature and pressure (30°C and 11.92 bar). At point F, the refrigerant is a saturated liquid.
Line F to A - represents the sub - cooling of the condensed liquid (from 30°C to 20°C).
REFRIGERATING EFFECT :
The amount of heat absorbed by each unit mass of refrigerant as it flows through an evaporator is known as the refrigerating effect, and is equal to the difference between the enthalpy of the vapour leaving the evaporator and the enthalpy of the liquid at the flow control.
Refrigerating effect (qE) = hc' - hA in KJ/kg.
REFRIGERATING CAPACITY :
The rate at which a system will absorb heat from the refrigerated space or substance is known as the refrigerating capacity.
Refrigerant Capacity, QE = m x qE KJ/S. where m = mass flow of the refrigerant. So, mass flow = QE \ qEin Kg/S.
COMPRESSOR CAPACITY :
The capacity of a compressor must be such that it removes the vapour from the evaporator at the same rate at which it is formed. To maintain a specified operating condition, a compressor must have a swept volume equal to the volume of vapour formed in the evaporator per unit time (m3/hr).
V = m x n m3
Where, n .= Sp. volume of the vapour at the compressor suction inlet in m3/kg at -25°C and 1.32 bar.
HEAT OF COMPRESSION :
The energy input to the compressor motor to raise the pressure of the vapour to the required condensing temperature is known as the heat of compression, and is equal to enthalpy difference of the vapour at the compressor outlet and inlet (Wc = hD - hc.) = Qc
CONDENSER DUTY:
The rate of heat transfer from the refrigerant to the cooling medium in the condenser. Qc = refrigerating effect + heat of compression = m (qE + Qc).
COEFFICIENT OF PERFORMANCE :
It is the ratio of the refrigerating effect i.e. heat removed in the evaporator to the work done in the compressor i.e. heat of compression:
REFRIGERANT :
PRIMARY REFRIGERANT -
Primary refrigerants are the working fluids used in vapour compression system. It is desireble for a primary refrigerant to be non - flammable, non - explosive and non - toxic, and it should not contaminate foods or damage the environment in the event of a leak.
a)
be non
- corrosive, and it should not react unfavourably with lubricants,
moisture
and materials used in plant construction.
b)
have moderate
working pressures. The condensing pressure should be as low
as
possible in order to keep down the mechanical strength required
in the compressor
and high pressure side of the system. The
evaporating pressure should be as
high as possible because
pressures below atmospheric result in air and moisture
being drawn
into the system in the event of a leak.
c) have a high COP value.
d)
have a
low'moderate temperature after compression. A low discharge
temperature
reduces the risk of oil decomposition and overheating
of the compressor.
e) have high latent of evaporation so that low refrigerant mass flow for a given duty,
f)
have low
specific jieat as it is desirable during throttling at the expansion
valve,
causes liquid refrigerant to be cooled at the expense of
partial evaporation.
h) High critical temperature of refrigerant, as it is impossible to condense at a temperature above the critical temperature, no matter how much the pressure is increased.
i) be low in cost and readily available.
MARINE REFRIGERANTS, THEIR CHEMICAL PROPERTIES -
R 11 - CCI3F - Trichloro.fluoro carbon R 12 - CCI2F2"- Dichloro difluoro carbon R 22 - CHCIF - Hydrochlorofluoro carbon R 502 - CHCIFJCF.C CIF9 R 717-Ammonia NH3
R 11 is used for marine air conditioning, especially for cruise ships, and for cleaning out marine refrigeration machinery.
R 12 is used for marine airconditioning and for food stores in ships and has universal use in refrigerated containers.
R 22 is used in marine airconditioning and food stores in newer ships and in most central cargo plant, fishing boat refrigerated storage and freezing plant and liquid gas tanker re-liquefication plant.
R 502 has very occasional use for low temperature refrigeration.
Ammonia is used for large freezing and low temperature storage installations.
CHEMICAL PROPERTIES :
R 11, R 12, R 22 and R502do not react with steel, copper, aluminium and brass, but attack lead, tin, zinc magnesium and their alloys. They also attack natural rubber, some elastomers and polytetrafluroethane. So it is important to ensure that the correct materials are used in gaskets, seals, jointing and packing.
Ammonia reacts with copper, zinc and their alloys, so steel only shuld be used in NH2 plants. It also attacks natural rubber and some elastomers. NH3 gas is extremely toxic, having pungent odour and can be fatal in enclosed space. It is inflammable in air at concentrations of 16% to 27% and may form a explosive mixture.