PROPERTIES OF MARINE FUEL:
Fuels are now a days purchased by quoting relevant ISO 8217, grade, in addition to mention any other qualities and conditions as may be desired by the buyer.
The most important properties of fuel are : density, viscosity, water content, flashpoint, sulphur content, Carbon Residue content, Ash content, vanadium content and specific energy content. Other properties such as distillation range, and cetane number are relevant to distillate fuels.
(1) DENSITY AT 15°C -
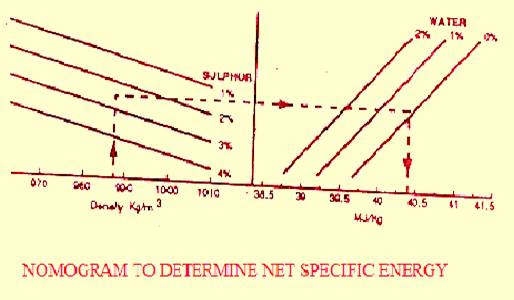
Density defines the mass per unit volume and expressed in Kg/m3 in SI units. Marine fuel oils have a linear density/temp, relationship, as opposed to water, for example, and decreases with increasing temperature at a rate of about 0.66 Kg/ m3 per degree °C rise. (Relationship is shown in the graph)
Significance :-
Quantity calculation
Separability of water and solids - to select the optimum size of gravity disc for purification.
Specific energy calculation.
Calculated
Carbon Aromar city Index (CCAI) - measures the ignition
quality
(increased index no. causes an increase in ignition
delay).
(2) VISCOSITY -
Viscosity of a fluid is the measure of its internal resistance to shear or flow and is measured in centistokes (cst) with a quoted reference temp. For distillate fuel it is expressed at 50°c and for residual fuel it is expressed at either 50°c or 100°c.
Significance :-
Fluidity & heating needs for transfer, separation & injection.
(CCAI) Calculation (ignition performance)
Purchasing grade paid for.
Viscosity itself is not an indication of fuel quality, but since viscosity decreases as they are heated, knowledge of viscosity change with temperature is required to make it possible to estimate the temp, at which fuel is to be stored for pumping at the most convenient rate. It is/also necessary to gauge the temp, at which residual fuels are to be atomised as recommended by the engine manufacturer. Final control of fuel heating should be viscometic rather than thermostatic. By means of Viscometer in the fuel line, the final viscosity of a blend of two different grades may be readily obtained before the mixture is delivered to the engine.
It has been noticed that pruchasing 180 cst at 50°c blended oil is not necessarily better than 380 cst oil. This is because of inclusion of cycle oil, slurry oil as cutter stock to produce the blend resulting carry over of catalytic fines, which can cause much harm to engine parts. In general it is advisable to purchase 380 cst fuel oil provided the vessel has the capability of sufficiently heat the oil to obtain the required viscosity for atomisation.
Kinematic Viscosity - It is the ratio of the absolute viscosity to the density.
The base units of kinematic viscosity is m2/sec but this is coarse for practical use and the centistoke is used with unit of-mm2/sec.
Older practice was to use the Redwood system of measurement, which used units of seconds and was a measure of the time for a sample of oil to flow through a standard orifice at a specified temperature.
For efficient atomisation (and hence combustion) oil should be heated so that oil viscosity comes down in the range of 65 to 75 Redwood No. 1 or 15 to 18 cst.
(3)
WATER CONTENT -
Significance
:-
Wet sludge
Corrosion (especially sea water)
Possible
ignition interference
Displacement of fuel quantity
Specific energy calculation (heat loss)
Saline Water in the emulsified state has a particular affinity for highly cracked fuels and heavy sludging can be experienced during purification. Water can cause cavitation at pump suction, corrosion and while in combustion space, atomisation can be disturbed, ignition retarded and the cylinder lubricant film diluted.
The ingress of water can come from tank condensation and steam pipe leakage where steam is used for tank heating purposes. Water is normally removed by gravitational separation in the fuel tanks and by the centrifuge purification system.
(4)
ASH CONTENT -
Significance
:-
Inorganic incombustible materials, that are metals may form deposits on piston rings, grooves, liners,' exhaust valves, turbochangers etc.
Specific energy calculation, (heat loss)
The sources of ash forming materials may be divided into three distinct groups -
(a) Organic metal compounds existing naturally in the crude stock and concentrated into the residual fraction during refining, principally vanadium although from the very nature of the crudes formation most elements will be present including aluminium, silicon, sodium, iron, calcium at limited levels.
(b) Materials introduced deliberately during refining process principally aluminium and silicon as catalytic fines and spent lubricant dumped into marine fuels (mainly lead, zinc and calcium)
(c) Materials introduced inadvertently during storage, transfer or delivery, such as scale and rust (iron), saline water (sodium) or earthly matters (silicon).
(5) FLASH POINT - Statutory Safety requirement
The flash point of a fuel is the lowest temperature at which sufficient vapour is given off to produce a flash on application of a flame under specified test conditons. The flash point is measured by Pensky - Marten apparatus under closed cup test method for marine fuels. The minimum flash point for fuel in the machinery space of a merchant ship is governed by international legislation and the value is 60°c. For fuels used for emergency purposes, external to the machinery space, such as in lifeboats, the flash point must be greater than 43°c. The purpose of defining a minimum flash point is to minimise fire risk during normal storage and handling. Classification requires that the fuel should not be heated to within 10° of the oil F. R It is also an indication of the extent of lighter fraction present in the residual oil.
(6) POUR POINT - Wax formation impeding fluidity.
The pour point is the lowest temperature at which a marine fuel oil can be handled without excessive amounts of wax crystals forming out of solution. At 3°c below pour point temperature, the fuel will become get thereby preventing flow under its own weight. So more viscous fuel are required to be heated to maintain their pumpability.
(7)
SULPHUR -
Significance
:-
Low temperature corrosion wear of cylinder liners and rings. Adequate lubrication with alkaline lubricant and controlled cylinder temperature.
Specific energy Calculation (heat loss)
Sulphur is a naturally occuring element in crude oil and varies from 0.5 - 5% by weight. Reducing the amount of sulphur in refinery-is very expensive. Desulphurisation can push up bunker cost by up to US$ 50/Tons.
Sulphur in the fuel combines with oxygen to form SO2 and SO3. Sulphur trioxide reacts with moisture, assisted by the presence of vanadium as a catalyst, forms sulphuric acid vapours and where the metal temperature are below the acid dew point (160°c), the vapours condense as sulphuric acid, resulting in corrosion.
Modern TBN lubricants and maintaining temperature in the engine above dew point can avoid corrosion. However high sulphur content can reduce nett calorific value (specific energy) by about 1% over a low sulphur fuel.
Sulphur dioxide from the combustion process is an air pollutant, additionally it can cause "Cold End" corrosion.
2SO2
+ O2
---►2SO3---►V2O5
Catalyst
SO3 + H2O
---► H2SO4
This process relies on condensation, hence both the partial pressure of the water vapour and the temperature of the exhaust gas is important. The presence of free oxygen is required.
Reducing the excess air in the combustion process helps in retarding :~e formation of the damaging acid, also helps in avoiding the catalyst action.
(8) CARBON - RESIDUE -
Significance :-
Indication of tendency to formation of carbonaceous deposits. However, corelation to field performance is poor.
The carbon residue of a fuel is the tendency to form Carbon deposits in the combustion chamber and exhaust system and may be expressed as either Ramsbotton Carbon Residue (distillate fuel), or Conradson Carbon Residue (residual fuel) or micro - carbon residue.
It is a measure of carbonaceous residue remaining after destructive distillation of a sample of oil. It gives an indication of the graphitic carbon forming tendency and is a guide to the rate of fouling, piston ring sticking in diesels and slagging in boilers.
(9) CATALYTIC FINES (SILICON AND ALUMINA)
These Catalytic fines are particles arising from the catalytic cracking process in the refinery and are in the form of complex alumino - silicates. These are very hard and abrasive and if present in sufficient quantity, could result in high wear in various engine components. The ISO 8217 standard requires the aluminium + silicon content to be maximum 80 mg/kg. Centrifuging a fuel containing this maximum level can be reduced to 15 mg/kg. by purification on board..
(10) VANADIUM -
Vanadium is a metal that is present in all crude oils in an oil soluble form and the levels found in residual fuels depend mainly on crude oil source.
With vanadium/sodium ratio of 3:1, high temperature corrosion after combustion, especially in exhaust valves. Not removable by on-board treatment.
A hard, white metallic element, density 5500 kg/m3.
Melting point of the pure metal 1710°C.
In fuel oil, it is tied up in the co-valent bond structure of the hydro-carbon, this means it can not be removed easily.
In the combustions process, it can readily combine to form a variety of "Low Melting point" compounds, typical of which are :-
Sodium Metavandate. Na2O - V2O5 Melting point 630°C
Sodium Vandate. Na2O - V2O4 - % V2O5 M.P 630°C
Vanadium Pentaoxide. V2 O5 M.R 670°C
These compounds, when in the liquid state, can do a considerable amount of damage by liquid metal attack. The main effect being from the vanadium. This corrosion is rapid with steels but no metal is immune. The process is commonly referred to as "High temperature corrosion."
In the solid state the vanadium compounds adhere to the metal surfaces, forming needle like deposits. The build up can be very rapid. The thermal conductivity of the deposits is very low and this can affect the heat transfer in boilers, a build up of these deposits can mis-direct gases. Dust from vanadium deposits is an irritant to the respiratory system, it is necessary to provide protection when clearing.
11) SODIUM :
High temperature corrosion at 3:1 V/Na ratio.
Can
contribute significantly to ash deposits, in post combustion period
in way
of turbochargers.
Sodium in the form of NaCI present in seawater contamination can normally be removed by centrifuging.
12) IRON :
Rust
and scales may be abrasive and if so,
should be removable by
centrifuging.
14) CALCIUM (Ca)
May be indicator of waste lubricants, but also inherent in a few crude oils.
Frequently used as additive to lubricants for the purpose of neutralising acidic componets from the combustion process (sulphuric acid).
15) MAGNESIUM (Mg)
Main source is seawater contamination.
16) LEAD (Pb)
Used
as additive in petrol and hence when found in fuels may indicate
the
present of waste lubricants.
17) ZINC (Zn)
Typical
antiwear additive used in lubricants and hence indicates the
presence
of waste lubricants.
(18)VOLATILITY :
It
is the measure of the tendency for a hydrocarbon to vaporise. A
mixture of hydrocarbon vapour and air (in the flammable limits) is
explosive.
Volatility values are to give guidance for oil storage
and heating. The standard test
for volatility is Flash and Fire
points measured with Pensky-Marten test.
(19)CALORIFIC VALUE :
The heat-value of carbon is 34MJ/kg and for hydrogen it is 122MJ/kg, hence for molecules with same number of Carbon atoms the Paraffin molecules with its greater number of hydrogen atoms will have a larger heat release than the equivalent Naptha or Aromatic molecules.
The paraffin molecule is large than the corresponding Naptha or Aromatic, so there are less of them per unit volume, i.e. the density of corresponding Napthas and Aromatics is greater than the Paraffin.
Hence there is a relationship between specific gravity and calorific value, the fuels with the lower S.G. are having the higher calorific value. The Calorific value is established by Bomb Calorimeter.
