![]()
HEAT TREATMENT OF STEEL
Heat treatment of metals may be defined as combination of operations involving the heating and cooling of metals or alloys in the solid state to produce certain desirable mechanical properties.
The heat treatment processes may be considered to consist of three main parts:
1) The heating of the metal to the pre-determined temperature.
2) The soaking of the metal at that temperature until the structure becomes uniform throughout the section.
3) The cooling of the metal at some pre-determined rate to cause the formation of desirable structures within the metal for the desired properties.
Purpose and Methods of Heat Treatment :
PURPOSE
a) To improve machinability.
b) To relieve internal stresses.
c) To improve mechanical properties such as ductility, strength, hardness, toughness etc.
d) To change the grain size.
e) To increase resistance to heat and corrosion.
f) To modify electrical and magnetic properties.
g)
To change the
chemical composition.
h) To remove gases.
PROCESSES
1) Annealing
2) Normalising
3) Hardening
4) Tempering
5) Case Hardening
a) Carburising
b) Cyamiding
c) Nitriding
6) Surface Hardening
a) Induction hardening
b) Flame hardening
7) Diffusion Coatings
1) ANNEALING :- Purpose of annealing is to obtain one or more of the following effects-:
a) Soften the steel
b) Improve machinability
c) Increase or restore ductility and toughness.
d) Relieve internal stresses.
e) Reduce or eliminate structural inhomogeneity.
f) Refine grain size.
g) Prepare steel for subsequent heat treatment.
FULL ANNEALING -
Full annealing consists of : (1) heating the steel slightly above the critical Point (2) holding it at this temperature for a considerable period, and (3) slowly cooling.The annealing temperature is 30°c to 50°c above upper criticajline for hypoeutectoid steels (less than 0.83%c) and about the same amount above lower critical line for hypereutectoid steels (more than 0.83%c). After the required heating temperature is attained, the metal is held at that temperature for a certain time to enable the internal changes to take place throughout the mass of the workpiece. The holdiing time at the annealing temperature is not less than 3 to 4 minutes for each millimeter of section of the largest piece being treated.
The metal is then slowly cooled either holding in the furnace or buried in a non conducting material such as sand, lime or ashes. Carbon steels are cooled down at a rate of 150°c to 200°c per Hour, while alloy steels, in which austentile is very stable, should be cooled much slower (30°c to 100°c per hour).
This slow cooling is required in annealing to enable austentite to decompose at low degrees of supercooling, so as to form a pearlite and ferrite structure in hypoeutectoid steel, a pearlite structure in eutectoid steel and a pearlite and cementite structure in hypereutectoid steel. In a successful annealed steel, the grains of ferrite are large and regular while the pearlite consists of coarseplates of cementite and ferrite.
Hot worked steels (rolled stock, sheet, forgings etc) as well as castings of carbon exhibit a coarse structure due to which weakness and brittleness results. Annealing effectively breaks up this coarse as-cast structure and replace it by fine-grained material. While the tensile strength is not greatly affected by the treatment, both toughness and ductility are improved.
![]()
PROCESS ANNEALING -
When a steel is cold worked the hardness and elastic properties considerably increase, while the ductility remarkably suffers and steel becomes unsuitable for further plastic deformation. The ductility of steel may then be restored by so-called recrytallisation or process annealing. Furthermore, the effect of process annealing is to relieve internal stresses resulting from any previous heat treatment.
Process annealing consists in heating the steel to a temperature below lower critical line, usually 500°c to 700°c, holding at this temperature for a prolonged period in a batch-type or continuous furnace usually with an inert atmosphere of burnt coal gas, and slow cooling. This causes recrystallisation of crystals from a broken up or distorted state and ductilily improves. In this process, carried out at below lower critical temperature, although recrystallisation is promoted, there is no phase change.
SPHEROIDISE ANNEALING
The aspheroidised condition is produced by annealing the steel at a temperature of 650 - 700°c, just below the lower critical line (723°c), holding at this temperature for sometime then followed by slow cooling in the furnace at the rate of 25°c to 30°c per hour to a temperature of 550°c to 600°c. The subsequent cooling may be conducted in still air at room temperature. This process transforms lamellar pearlite into globule type.
The range of heating temperature at which globular pearlite is formed, is very narrow in steels with near- eutectoid compositions. The small globules will not only improve the surphase finish-during machining but will also be dissolved more quickly when the tool is" ultimately heated for hardening.
HOMOGENISING
Homogenising or diffusion annealing is applied to steel ingots (both carbon and alloy steels) and heavy complex castings for eliminating chemical inhomogeneity within the separate crystals by diffusion.'
This is carried out at a temperature from 1100°c to 1200°c (the optimum temperature is 1150°c) at which diffusion proceeds quite easily and to some extent, equalises the composition of steels. After the required heating temperature is achieved, the metal is held for a very short period. Holding is followed by cooling with the furnace for 6 to 8 hours to 800°c to 850°c and then further cooling in air.
Homogenising naturally causes a very rapid growth (for heating to a very high temperature) of austenitie grains. After homogenising, therefore, the steel ingots necessarily undergo an ordinary phases (full), annealing for the fine-grain structure to be formed.
ISOTHERMAL ANNEALING -
Isothermal annealing is carried out as for ordinary annealing to form austentite. It is then cooled comparatively rapidly in air or by a blast in a furnace to a temperature of 50°c to 100°c below lower critical line, i.e. 600°c to 700°c. The steel is held isothermally at this temperature (at constant temperature) during a certain period of time to provide for complete decomposition to pearlite and hence the name isothermal annealing. This is followed by comparatively rapid cooling.
A homogeneous structure of the metal is obtained and the results of annealing are somewhere found more stable. The main advantage of isothermal annealing is that it reduces the time required for heat treatment of the steel. This is specially true for alloyed steels which must be cooled very slowly to obtain the required reduction in hardness.
2) NORMALISING :-
Normalising is similar to annealing in all respects save and except two - the heating range which is about 40°c to 50°c above that of annealing and the rate of cooling is increased by allowing the metal to cool in air.
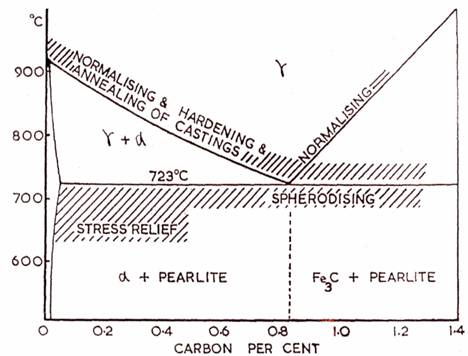
Heat-treatment Temperatures of Carbon Steels in Relation to the Equilibrium Diagram.
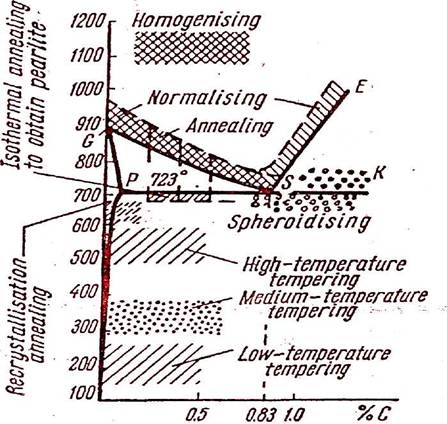
Temperature ranges for various heat treatment processes
Normalising is preferred if the improvement of mechanical properties is the main objective because rapid method of cooling limits grain growth as well as the surface finish of a normalised article is often superior to than of an annealed when machined, since the high ductility of the annealed article often gives rise to local tearing of the surface. Consumption of fuel or electric power is more in annealing process than normalising. However, where considerable relief of internal stresses and machinability are main objective, annealing is preferred over normalising.
Normalising is used particularly for the following purposes :
1) To eliminate coarse-grained structure.
2) To remove internal stresses that may have been caused by working.
3) To improve mechanical properties of steel.
In addition to all these, it may be used to increase the strength of medium carbon steels to a certain extent, to improve machinability of low carbon steels, to improve the structure of weld etc.
The process of normalising consists of heating the metal to 40°c to 50°c above upper critical line, holding at this temperature for a short time (about 15 minutes) and cooling in air. This produces a homogeneous structure consisting of ferrite and pearlite for hypoentectoid steel, only pearlite for entectoid steel and pearlite and cementite for hyperentectoid steels, the normalised structure of alloy steels consists of ferrite and sorbite.
Normalised steel have a higher yield point, higher tensile strength and impactstrength than if they are annealed, but ductility and machinability obtained by normalising will be somewhat lower.
When steel is cold-worked, the crystal structure" is distorted and the metal may be brittle. The internal structure of hot worked forged part may also be distorted owing to being worked at a very low temperature. Likewise, if a casting is poured at a somewhat indefinite temperature and cools at different rates in different parts, it may be unreliable. In all the above cases normalising can eliminate the defects.
HARDENING.
The purpose of hardening with subsequent tempering are :-
1) To develop high hardness to resist wear and to enable it to cut other metals.
2) To improve strength, elasticity, ductility and toughness.
The process consists of: (a) heating the steel to a temperature above critical point,(b) holding at this temperature for some period, and (c) quenching (rapid cooling) in water, oil or molten salt bath.
Hypoentectoid steels are heated from 300c to 500c above higher critical temperature while hyperentectoid steels are heated about the same amount above lower critical temperature. In the first case, ferrite + pearlite and in the second case, pearlite + cementite are transformed into austentite upon heating. A considerable part of cementite is retained. Rapid cooling should enable the austentite to be supercooled to the martensite point.
Alloy steel an high speed steels are heated for hardening to about 1100°c to 1300°c and cooled in a current of air. The alloying element increase the stability of austentite and retard the decomposition of austentite into martensite. That means with less drastic speed of cooling the same harder martensite constituent can be obtained as would be developed in a carbon steel by a faster rate of cooling.
Requirements for hardening :
a)
COMPOSITION, CARBON AND ALLOY
CONTENT -
Steel with low carbon content will not respond to hardening
treatments, because ferrite present is soft and not changed by the
treatment. As the carbon content increases, the possible hardness
obtainable also increases. Transformation of austentite to pearlite
is rapid and easily
accomplished during ordinary cooling at room
temperature due to rapid diffusion of carbon in the face-centred
cubic lattice and allotropic transformation. With extreme rapid
cooling rates a phase known as Martensite is obtained, which may be
considered as a supersaturated solid solution of carbons in
body-centred cubic iron Martensite
is very hard and brittle. Less
severe quenching gives rise to Bainite or Troostite. The hardening
temperature of steel to form homogenous austentite depends upon its
chemical composition and upon its carbon content.
HEATING
RATE AND HEATING TIME AND QUENCHING TIME, MASS EFFECT
-
Compositions,
size and shape of the component to be hardened, dictate the rate at
which it shall be cooled. Large masses of steel of heavy section will
obviously cool more slowly than small articles of thin section when
quenched, so that while the surface skin may be martensitic, the core
of a large section may be bainite because
it has cooled more
slowly. If, however, small amounts of such elements as nickel,
chromium or manganese are added to the steel, it will be found that
the martensitic layer is much thicker than with a plain carbon steel
of similar carbon content and dimensions which has been cooled at the
same rate. Alloying elements therefore
increases the depth of
hardening and they do so by slowing down transformation rates. Thus
alloy steel to be hardened requires less drastic quenching method
than are necessary for plain carbon steels.
QUENCHING MEDIA, TEMPERATURE AND DEGREE OF AGITATION -The quenching media is chosen according to the rate at which it is desired to cast the steel. The following list of media is arranged is order of quenching speeds :-
5% Caustic Soda (NaOH)
5 - 20% Brine (Nacl)
Cold Water
Warm Water
Mineral Oil
Animal Oil
Vegetable Oil.
The very drastic quench resulting from the use of caustic soda solution is used only when extreme hardness is required in components of simple shape. For more complicated shapes an oil-quenched alloy steel would give better results. The flash point, viscosity and stability of oil are to be considered for the choice of a quenching medium.
Hardenability i.e. the depth and distribution of hardness depends on the quenching medium and method of quenching, composition of steel, grain size and alloying elements.
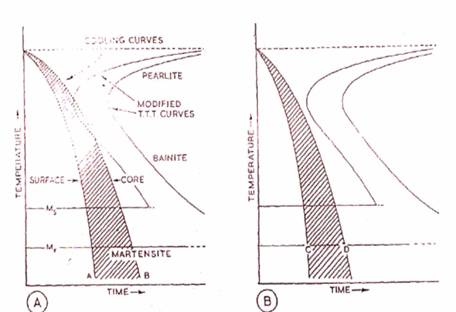
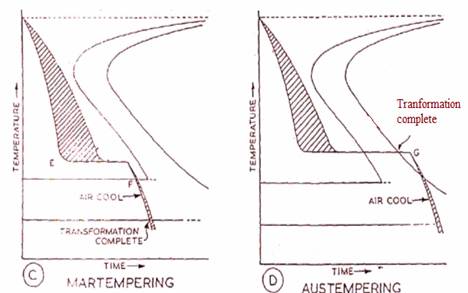
(A) and (B) Illustrate the Effects of Mass During Normal Quenching. (C) and (D) show how these effects may be largely-overcome in martempering and austemoering.
MARTEMPERING -
Martempering or interrupted quenching is a hardening operation that produce's martensite. It is not tempering. In this method steel is heated to the hardening temperature and then quenched in a medium, usually in a salt bath, having a temperature, just above that where martensite starts to form (usually from 150°c to 300°c). The article is held until it reaches the temperature of the medium but not long enough to austentite decomposition. It is then cooled further to room temperature in air and sometimes in oil. The austentite is transformed to martensite during the last period of cooling to room temperature. The treatment will provide a structure of martensite and retained austentite in the hardened steel.
Martempering has the following advantages over conventional quenching - (1) less volume changes occur due to the presence of a large amount of retained austentite, (2) less warping since the transformation occurs almost simaultaneously in all parts of the article and (3) less danger of quenching cracks.
AUSTEMPERING-
In austempering or isothermal quenching the steel part is heated to the required hardening temperature and then quenched in a molten salt or lead bath usually at a higher temperature i.e. from 300°c to 350°c. The steel is held in the bath for as long a time as is needed for isothermal transformation of the austentite, i.e. until transformation to bainite (troostite) and not martensite is complete. Although the steel is of the same hardness as that of martensite, it is tougher and more ductile than other quenched and tempered steels. Tempering is rarely needed after austempering.
3) TEMPERING :-
Steels that have undergone hardening quench, are usually mixtures of austentite and martensite, with the latter constituent predominating. Both these structure, are unstable and slowly decompose and liable to develop quench cracks if aged at room temperatures, with martensite present which is very brittle and hard.
The primary object of tempering are :-
- 1) To stabilise the structure of the metal, by separation of the carbon atoms from the space lattice of tetragonal martensite. (Between 80° to 200°c)
2)
To reduce internal stresses produced during previous heating
by decomposition
of residual austentile. (200° to 300°c)
3)
To reduce some of the hardness produced during hardening and
to increase the
ductility of the material, by transformation into
cementite. (300° to 400°c)
4)
To give the metal right structural condition combined with
toughness and shock-
resistance, by coagulation of cementite.
LOW TEMPERATURE TEMPERING -
It is done in the temperature range from 150°c to 250°c. The purpose is to relieve internal stresses and to increase the ductility without appreciably reducing its hardness. Low temperature tempering is applied in the heat treatment of Carbon steel and low alloy steel cutting tools as well as measuring tools.
MEDIUM TEMPERATURE TEMPERING -
It is done by heating the metal to 350°c to 450°c. The structure of steel is altered martensite is transformed into secondary troostite. The results are a reduction in hardness and strength of the metal and an increase in the elongation and ductility. It is mainly applied to parts which are subjected to impact loads : chiesels, hammers, springs etc.
HIGH-TEMPERATURE TEMPERING - It is done in the range of 500°c to 650°c. At this temperature Sorbite is formed in thesteel and the internal stressers are almost completely eliminated. High temperature tempering imparts high ductility to parts, yet permits them to retain adequate hardness. This is applied to machine parts which are subjected to high stresses and impacts : Shafts, connecting rods etc.
CASE - HARDENING :-
a) CARBURISING - Usually low carbon steel of about 0.15% carbon is used which does not respond appreciably to heat treatment. In course of this process, the outer layer is converted into a high carbon steel with a carbon content ranging from 0.9 to 1.2% carbon. If it receives proper heat treatment, it will have an extremely hard surface on the outside and a soft ductile core.
The process consists of heating iron or steel to red heat (900 - 950°c) in-contact with some carbonaceous materials upto 5 hrs such as wood, bone or charcoal with compound such as carbonates of barium, calcium or sodium which are termed "energiser". These energisers are added with the material to increase the concentration of carbon monoxide and thus improve the rate of carburising. Carbon enters the metal to form a solid solution with iron and converts the outer surface into a high carbon steel.
Energiser is a mixture of sodium Carbonate and barium Carbonate (40% of total composition) and its purpose is to accelarate the solution of carbon by the surface layer of steel.
Case hardening makes use of the fact that carbon will diffuse into iron provided that the latter is in the austentite form which exists above 910°c. Carburisation proceeds with the dissociation of carbon monoxide in contact with the hot steel. The atomic carbon deposited at the surface of steel dissolves easily in the metal. At the same time Barium carbonate reacts with some of the carbon from the charcoal, liberating more carbon monixide. Barium oxide then combines with Co2 and forms Barium Carbonate. The cycle of reaction is same for sodium carbonate.
5) CYANIDING -
It is a process of producing hard surface on low and medium carbon steel in a molten salt bath containing cyanide maintained at 870 - 950°c for 15 minutes to 1 hour to get surface hardness.
Cyanide bath contains one third of each of sodium chloride, sodium carbonate and sodium cyanide.
2NaCN + 2O2 = Na2CO3 + N2 + CO
The nitrogen formed dissolves in the surface and produces an increase in hardness by the formation of nitrides. Main advantage of cyanide hardening is the pyrometric control of the liquid bath. Moreover after treatment the basket of work can be quenched.
Cyanides are extremely poisonous and every precaution must be taken to avoid inhaling the fumes from the pot.
6) NITRIDING -
It is a process of producing hard surface layer on alloy steel only. Process consists of heating the steel in an atmosphere of ammoia gas at temperature of 500°C to 650°C without further heat treatment. NH3 is dissociated and nascent nitrogen combines with elements in the steel to form Nitrides. These nitrides gives extreme hardness to the surface. A hard surface layer usually from 0.2 to 0.4 mm in depth is produced in 50 hours.
NH3
![]() 3H
+ N
3H
+ N
For the Nitriding process, special alloy steels are necessary, since hardening deposits on the formation of nitride are of such metals as aluminium, chromium and vanadium. Nitriding a plain carbon steel would fail because the iron nitrides formed would diffuse to a considerable depth, so that the surface hardness would be lost. Aluminium nitride, chromium nitride and molybdenum nitride give case hardness.
Before being nitrided, the components are heat-treated to produce the required properties in the core. .
ADVANTAGES OF NITRIDING -
a) Since no quenching is required after nitriding, cracking or distortion are unlikely and components can be machine-finished before treatment,
b) A very high surface hardness of upto 1150 V.RN is obtained with the "Nitralloy" steels (Aluminium type).
c) Resistance to corrosion is good if the nitrided surface is left unpolished.
d) Resistance to fatigue is good.
e) The hardness is retained at elevated temperatures upto 500°c, whereas in a carburised component, hardness begins to fall at about 200°c.
f) The process is cheap when large numbers of components are to be treated.
DISADVANTAGES OF NITRIDING -
a) The initial outlay for plant is higher than with case-hardening, so that the process is economical only when large number of componints are to be treated.
b) If the nitrided component is accidentaly overheated the surface hardness will be lost completely, and the component must be nitrided again.
7) INDUCTION HARDENING -
Surface hardening of crankshafts, camshafts axleshafts etc. are done by an extremely rapid heating and quenching without effect on the interior core.
A high frequency current of about 2000 Hz is passed through a copper inductor block which acts as a primary coil of a transformer. The block is placed around but do not touch the surface to be hardened. Heating effect due to induced eddy current and hysteresis loss raises the temperature to about 750°c to 760°c for 0.5% carbon steel and 790° - 800°c for alloy steel. Hardened areas are then quenched immediately by sprays of water. A depth of case hardening of 3 mm is obtained in about 5 seconds.
8) FLAME HARDENING -
The process consists of heating steel with oxy-acetyleue flame at the surface which needed surface hardening. The surface is heated to above its upper critical temperature and immediately quenched by a water-jet attached to the torch. Only steels with a carbon content above 0.4% can be hardened effectively, though small amounts of nickel (upto "4.0%) and chromium (up to 1.0%) can be added with advantage. Before hardening, the components are generally normalised so that final structure will consist of martensitic case about 0.15 inch thick, and a tough ferrite/pearlite core.
9) DIFFUSION COATING -
Diffusion Coating or metallic cementation is the process of impregnating the surface of steel with aluminium, chromium, silicon, boron, beryllium and other elements.
Diffusion Coating is accomplished by heating and holding steel parts in direct contact with one of the above elements which may be in the solid, liquid or gaseous state.
The process imparts a number of valuable properties to steel, among which are high heat, corrosion and wear resistance.