HEAT-TREATMENT OF PLAIN CARBON STEELS
The three important phases of steel are namely, austentite (y), ferrite (a) and cementite ( Fe3C). Austentite is the solid solution which is formed when carbon dissolves in face-centred cubic iron, while ferrite is the very weak solid solution produced when carbon dissolves in body centred cubic iron below 910°c. Cementite is a carbide, Fe3C, formed by combination of carbon with some of the iron.
Plain carbon steel containing 0.3%.carbon, when begins to solidify at about 1,515°c, it will start forming dendrites of the solid solution 5. These dendrites will develop and change in composition due to diffusion promoted by the slow rate of cooling, unttf at 1492°c, they will be of composition C (0.08% Carbon). The remaining liquid will have become correspondingly enriched in carbon and will be compositon B (0.55% Carbon). At 1492°c, a peritectic reaction takes place between the remaining liquid and the 6 -phase, resulting in the disappearance of latter and the formation of austentite of compositions P (0.18% Carbon). As the temperature continues to fall the remaining liquid solidifies as austentite, and when solidification is complete the structure will consist of coned crystals of austentite of overall composition 0.3% Carbon.
Since the cooling is slow, diffusion of carbon will be promoted, and by the time we reach the line FE, the structure will consist entirely of crystals of austentile of practically uniform Carbon content. Moreover, due to the considerable time that the casting remained at high temperatures, grain growth will have been excessive, leading to formation of very large austentite crystals, particular in the centre of the casting.
The upper critical temperature of steel Varies with carbon content and is that temperature below which austentite begins to transform to ferrite and cementite under conditions of slow cooling. It will thus be represented by a point on FEG. In the case of steel under consideration the upper critical temperature will be represented by F and as the temperature falls below it, the face centred cubic structure becomes unstable and crystals of ferrite begin to separate out from the austentite. Since this ferrite contain rather less than 0.03% carbon, the carbon content of the remaining austentite will increase progressively as more and more ferrite is formed, until, at 723°c (The eutectoid temperature), the structure consists of ferrite, containing 0.03% Carbon, and austentite containing 0.83% Carbon.
At 723°c, the remaining austentite transforms to the eutectoid, pearlite, by depositing alternate layers of ferrite and cementite. The temperature 723°c, at which pearlite is formed is cafled the lower critical temperature and is the same for carbon steels of all compositions, since the eutectoid temperature is constant i.e. HEJ is horizontal.
A 0.83% Carbon steel will begin to solidify at approximately 1470°c by depositing dendrite of austentic of composition R and when solidification is complete, the structure will consist of cored crystals of austentite of overall composition 0.83% carbon. As the steel cools slowly, the structure becomes uniform by diffusion and no further structural change will take place until the point (E) is reached. For a steel of this composition, the upper and lower critical temperatures coincide, and the austentic structure transforms at this temperature to one which is totally perlite.

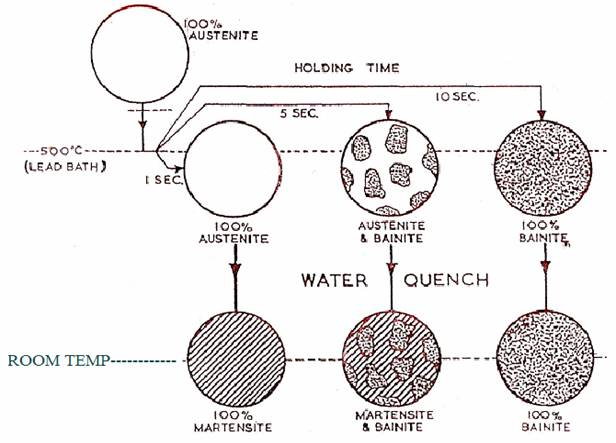
Diagrammatic Representation of the Method Used in Constructing T.T.T. Curves
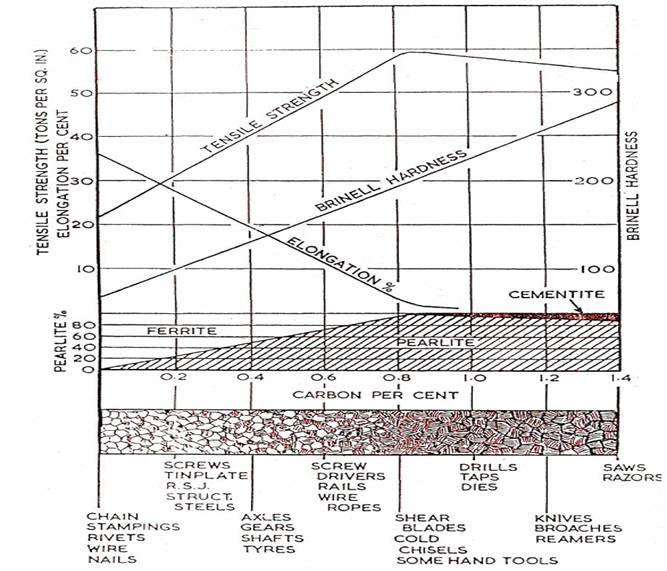
Diagram Showing the Relationship between Carbon Content, Mechanical Properties, Microstructure and Uses of Plain Carbon Steels in the Normalised Condition.
A 1.2% Carbon steel will solidify in a similar manner to the 0.83% alloy by forming austentite crystal of an overall carbon content of 1.2%. As the temperature falls to the upper critical for this alloy at G', needles of primary cementite begin to precipitate at the crystal boundaries of the austentite. Since cementite is being deposited, the remaining austentite will be rendered less rich in carbon, so that its composition will move to the left and when the temperature has fallen to 723°c, the remaining austentite will contain 0,83% carbon. As before, pearlite will now form, giving a final structure of primary cementite and pearlite.
Thus in a steel which has been permitted to cool slowly enough to enable it reach structural equilibrium, one of the following structure will form :
a) With less than 0.006% Carbon, it will be entirely ferritic. In practice such an alloy would be classed as commercially:pure iron.
b) With between 0.006% and 0.83% Carbon, the structure will contain ferrite and pearlite. The relative proportions of ferrite and pearlite appearing in the microstructure will vary according to the carbon content.
c) With exactly 0.83% carbon the structure will be entirely pearlitic.
With between 0.83% to 1.7% carbon, the structure will consist of cementite and pearlite, in relative amounts which depend upon the carbon content.