PLAIN CARBON STEELS - THERMAL EQUILIBRIUM DIAGRAM
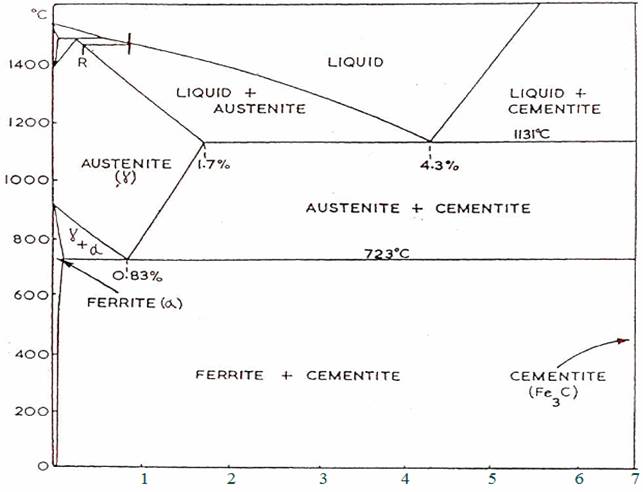
Plain Carbon steels are usually regarded as being those alloys of iron and carbon which contain up to 1.7% carbon.
There are several types of pattern or "space lattice" in which metallic atoms can arrange themselves on solidification, but the three most common are
(i) Body - centred cubic pattern
(ii) Face centred cubic pattern
(iii) Hexagonal close packed pattern.
Of these the hexagonal close packed represents the closest packing which is possible with atoms. The face centred cubig arrangement is also a close packing of the atoms, but body centred cubic is relatively "open"; and when, as sometimes happens, a metal changes its crystalline form as the temperature is raised or lowered, there is a noticeable change in volume of the body of metal. An element which can exist in more than one crystalline forms in this way is said to be allotropic. Thus pure iron can exist in three separate crystalline forms, which are designated letters "alpha" (ah "gamma" (y) and "delta" (5). Alpha-iron, which is body-centred cubic and exists at normal temperature, changes to gamma-iron, which is face-centred cubic, when heated to 910°c. At 1400°C, the face-centred cubic structure reverts to body-centred cubic delta-iron. (The essential difference between alpha-iron and delta-iron, is only in the temperature range over which each exists). These allotropic changes are accompanied by changes in volume - contraction and expansion respectively.
This reversible frame formation lies in the fact that up to 1.7% carbon can dissolve in face-centred cubic iron, forming what is known as "solid solution", while in body centred cubic iron no more than 0.03% carbon can dissolve.
As a piece of steel in its face-centred cubic form cools slowly and changes to its body-centred cubic form, any dissolved carbon present in excess of 0.03%, must be precipitated.
The solid solution formed when carbon dissolves in face-centred cubic iron is called Austentite, and the very weak (0.03% C) solid solution formed in the body-centred cubic structure is called ferrite. Ferrite is regarded to have almost the same properties as that of pure iron.
Symbol
'![]() '
(gamma) is used to denote both the face-centred cubic form of iron
and the solid solution austentite, while the symbol ‘
'
(gamma) is used to denote both the face-centred cubic form of iron
and the solid solution austentite, while the symbol ‘![]() ’
(alpha) is
used to denote both the body centred cubic form of iron existing
below 910°c and solid solution ferrite. When carbon is
precipitated from austentite, it is not in the form of elemental
carbon (graphite), but as the compound iron carbide (Fe3C)
usually called camentite. This substance like most other metallic
carbides, is very hard, so that, as th.e amount of Carbon (and hence,
of cementite) increases, the hardness of the slowly-cooled steel will
also increase.
’
(alpha) is
used to denote both the body centred cubic form of iron existing
below 910°c and solid solution ferrite. When carbon is
precipitated from austentite, it is not in the form of elemental
carbon (graphite), but as the compound iron carbide (Fe3C)
usually called camentite. This substance like most other metallic
carbides, is very hard, so that, as th.e amount of Carbon (and hence,
of cementite) increases, the hardness of the slowly-cooled steel will
also increase.
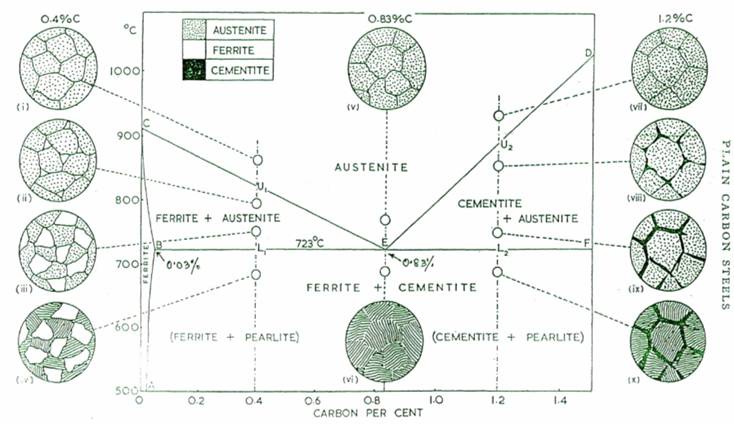
The thermal equilibrium diagram is in reality a chart which shows the relationship between the composition, temperature and structure of any alloy in a series.
On the extreme left of Iron-Carbon Thermal Equilibrium Diagram, is an area labelled "ferrite". This indicates the range of temperatures and compositions over which carbon can dissolve in body centred cubic (a) form of iron. On the left of sloping line AB, all
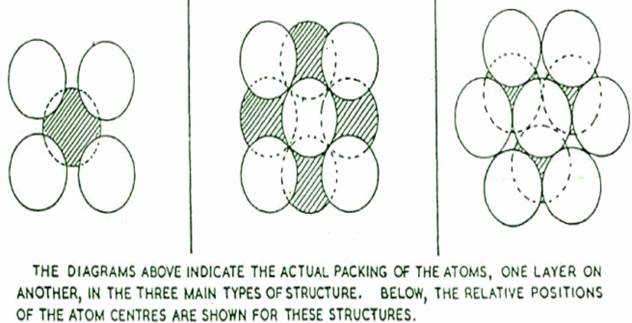
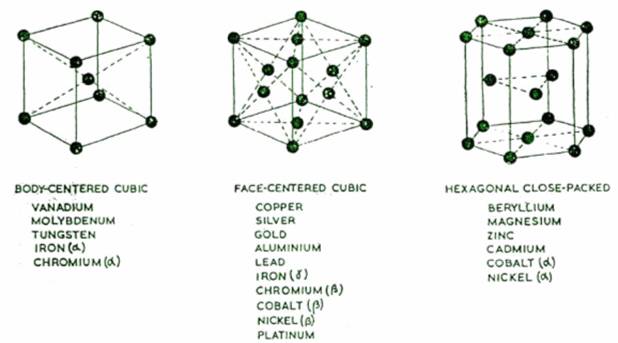
THE THREE PRINCIPAL TYPES OF STRUCTURE IN WHICH METALLIC ELEMENT CRYSTALLIZE
mg. 7.2. —Part of the Iron-Carbon Thermal-equilibrium Diagram.
carbon present is dissolved in the body-centred cubic iron, forming the solid solution ferrite, while any point representing a composition and temperature to the right of AB indicates that the solid solution a is saturated, so that some of the carbon contained in the steel will be present as cementite. The significance of the slope of AB is that the solubility of carbon in body-centred Cubic iron increases from 0.006% at room temperature to 0.03% at 723°c.
With the increase in carbon percentage, if a steel contains 0.4% carbon, and heated to some temperature above U1 (called upper critical temperature), it will become completely austentite. On cooling again to just below Ur the structure begins to change from one which is face-centred cubic to one which is body centred cubic. Consequently small crystals of body-centred cubic iron begin to separate out from the austentite. These body-centred cubic crystals containing a small amount of carbon (0.03%) are called ferrite crystals. As the temperature continues to fall, crystals of ferrite grow in size at the expense of austentite, and since ferrite is almost pure iron; most of the carbon present accumulates in the. shrinking crystals of austentite. Austentite can hold no more than 0.83% carbon in solid solution at this temperature of 723°c, represented by point E. As the temperature falls below 723°c at this stage, carbon begins to precipitate as cementite. At the same time, ferrite is still separating out, and the two substances, ferrite and cementite, form an alternate layers until all the remaining austentite is used up. This laminated structure of ferrite and cementite, then will contain exactly 0.83% carbon.
The above condition is termed as entectoid (when two metals which are completely soluble in the liquid state but completely insoluble in the solid state, they do so by crystallising out as alternate layers of two pure metals).
Any steel containing less than 0.83% carbon will transform from austentite to a mixture of ferrite and pearlite in a similar way when cooled from its austentic state. Transformation will begin at the upper critical temperature given by a point one which corresponds with the composition of steel and end at the lower critical temperature of 723°c. The relative amounts of ferrite and pearlite will depend upon carbon content of the steel, but in every case the ferrite will be almost pure iron, and the pearlite will contain exactly 0.83% carbon.
A steel containing 0.83% carbon will not begin to transform from austentite on cooling until the point E is reached. This transformation will begin and end at the same temperature (723°c). Since the steel under consideration contained 0.83% carbon initially, it follows that the final structure will be entirely of pearlite.
A steel which contains 1.2% carbon, will begin to transform from austentite when the temperature falls to its upper critical temperature at U2. Since the carbon in this time is in excess of the entectoid composition, it will begin to precipitate first, not as pure carbon, but as needle-shaped crystals of cementite round the austentite grain boundaries. This will cause the austentite to become progressively less rich in carbon, and by then temperature of 723°c bas_ been reached, the remaining austentite will contain 0.83% carbon. This remaining austentite will then transform to pearlite.
Any steel containing more than 0.83% carbon will have a structure consisting of cementite and pearlite, if it is allowed to cool slowly from its austentic state.
A plain carbon steel which contains less than 0.83% carbon is generally referred to as a hypo-eutectoid steel, while one containing more than 0.83% carbon is known as a hyper-eutectoid steel. Naturally enough, a plain carbon steel containing exactly 0.83% carbon is called a eutectoid steel.
CARBON PER CENT
The Iron-Carbon Thermal-equilibrium Diagram.
By very rapid cooling from the austentic condition, by water quenching, another structure, called Martensite is produced. Rapid cooling has prevented structural equilibrium from being reached and does not appear in equilibrium diagram, Martensite is very hard and very brittle, and used where extreme hardness is required. To increase the steel's toughness after quenching, at the expense of fall in hardness, the steel can be tempered. At tempering temperature in the region of 400°c, Troostite will form, while in the region of 600°c, Sorbite will be produced. Both Troostite and Sorbite can be regarded as extremely fine-grained pearlite.