FERROUS METALS
TYPES OF IRON ORE : Iron ores are generally carbonates, hydrates or oxides of the. metal, the latter being the best.
1. Magnetite (Fe3O4), Steel grey or black, which contains 62-72.4% iron when pure. It is a magnetic material - a fact which proves useful in locating deposits.
2. Red Hematite (Fe2O3), crystalline or granular, earthy or rock, red colour, contains 60 - 70% iron when pure.
3. Brown Hematite or Lipionite (2Fe2O3, 3H2O), brown, dense, earthy, contains 42-60% iron.
4. Siderite or Spathic (FeCO3), a shy grey, crystalline, contains 35 -48% iron.
5.
Iron Stone (FeCO3), grey to light brown, compact,
earthy or stony, contains 30
- 42% iron.
PRINCIPAL FERROUS METALS :
1. Pig Iron, 2. Cast Iron, 3. Wrought Iron, 4. Carbon Steet, 5. Alloy Steel.
PRODUCTION OF PIG IRON :
All iron and steel products are derived originally from pig iron. This is the raw material obtained from the reduction of iron ore in a Blast Furnace. The process of reduction of iron ore to pig iron is known as Smelting. The main raw materials required for pig iron are-(i) iron ore, (ii) Coking coal, and (iii) flux.
The coke used in the blast furnace should be a very high class hard coke obtained from good quality coking coals containing as low phosphorus and sulphur as possible. It is produced by heating what is commonly called "dry distillation" of the coking coals in coke ovens made of silica bricks in the absence of air to avoid giving off valuable gases. A light, porous, sufficiently firm fuel which burns well is thus obtained.
Flux is a mineral substance that is charged into a blast furnace to lower the melting point of the ore and to promote the removal of ash, sulphur and residues of the burnt fuel. Flux combines with the ashes of the fuel and the ore to form fusible products which separate from the metal as slag. The most commonly used blast furnace flux is limestone. Dolomite is less frequently used. They come straight from the mines. In some cases silicous alumina fluxes are charged into a blast furnace and in others, only silica.
PRE-SMELTING TREATMENT OF ORES :
Before the ore can be charged to the blast furnace preliminary treatment may be necessary in order to increase the efficiency of smelting.
a) Concentration - The removal of as much as posible of the earthy waste or gangue, which would otherwise take up useful space in the blast furnace, thus reducing output. Magnetic ores, such as magnetite, can be concentrated in a magnetic separator, whilst hematite ores are treated by washing, which removes much of the lighter earthy waste material.

b)
Calcination -
It is carried out in a kiln and is applied to those ores which
contain a large amount of moisture or carbon dioxide. These
substances may cause irregular working in the blast furnace and are
best removed before the ore is smelted.
Calcining also helps to
remove sulphur by oxidising it to sulphur dioxide. The ore is mixed
with coal and charged to the kiln, which works with natural draught,
this giving a sufficiently high temperature to expel water and carbon
dioxide.
c) Weathering - It is required if the sulphur content of the ore is high. The process involves stacking the mined ore in the open so that much of the sulphur present may oxidise and be washed away by rain.
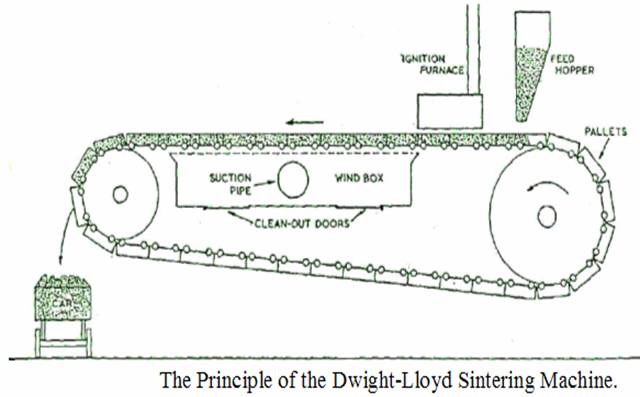
d)
Sintering -
The previous processes may
result in the
formation of a certain amount of dust. This dust is rich in iron, and
must therefore be reclaimed. It cannot be charged to the blast
furnace as it is, since it would tend to fill up the spaces
between
the lumps of the charge thus impeding the upward flow of gases or, be
blown out of the furnace. Hence, the dust is generally mixed with tar
or coal dust and treated in a sintering machine which produces lumps
of "sinter" of a suitable
size.
SMELTING OPERATION IN BLAST FURNACE :
The blast furnace consists of a vertical steel shell lined with refractory materials and having a charging arrangement at the top and a means of running off of pig iron and slag at the bottom, and designed for continuous operation. The smelting room of the blast furnace comprises of throat, stack, body, bosh and hearth.
The raw materials (alternate layers of ore, coke and flux), known as the charge, are taken to the top of the furnace (about 100ft high) by a specially designed bucket called "skip" running along an incline. The charge is then introduced into the throat of the furnace by means of a double bell and hopper arrangement to prevent escape of the blast furnace gas which is used as fuel. The proportion of raw materials are approximatelyone-half iron ore, one-third fuel and one-sixth flux.
To make 100 tonnes of pig iron from a good grade iron ore, the charge and products will be :
Charge . Products
Iron ore = 180 tonnes Pig iron = 100 tonnes
Coke = 95 tonnes Slag = 60 tonnes
Limestone = 50 tonns Gas = 500 tonnes
Air = 350 tonnes
For production of 500 tonnes of pig iron from a low-grade ore, the quantities in both charge and products will be :
Charge Products
Iron ore = 1200 tonnes Pig iron = 500 tonnes
Coke = 500 tonnes Slag = 750 tonnes
Limestone = 300 tonns Gas = 3,250 tonnes
Air = 2500 tonnes
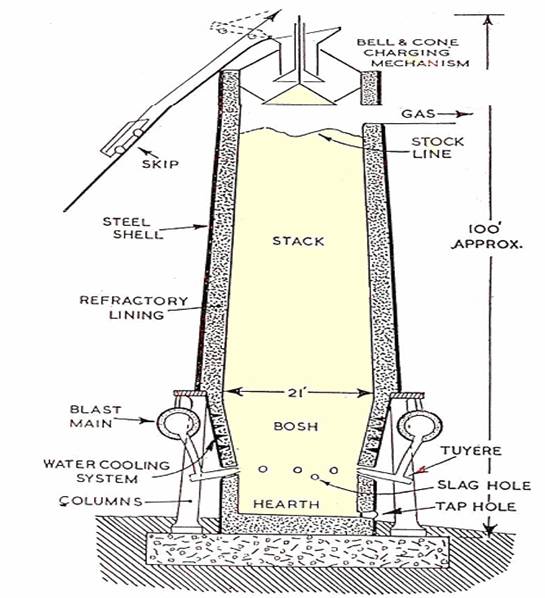
The Blast Furnace.
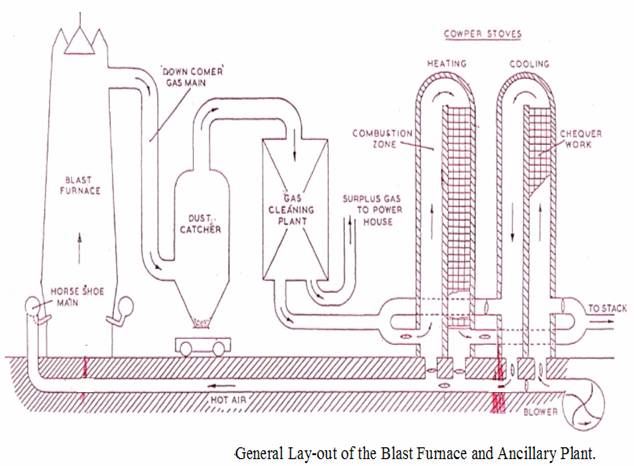
A hot blast of air is forced into the furnace through a number of nozzles called tuyers. The tuyers are cooled by water circulating between the pipe walls. The air blast is heated to minimise fuel consumption by passing the cold air blast through the heated checker-work hot blast stove.
During the production of pig iron large amounts of gas are evolved. This gas has a considerable calorific value due to. the carbon monoxide which it contains, and much of it is burned in chequered-brickwork stoves (cowper stoves) in order to preheat the air blast to the furnace.
The temperature of the furnace just above the level of the tuyers (melting Zone) being 1000°C to 1 700°c, all substances inside the furnace start melting in the heat. The limestone that serves as a flux combines with the ore to form a molten slag which floats on the top of molten iron. The slag is tapped off form the furnace through the slag hole. The molten iron is tapped at intervals from six to twelve hours through a tapping hole, the blast air being turned off meanwhile.
The air which is blown in at the bottom of the furnace causes partial combustion of the coke - 2C +O2 = 2CO + Intense heat As the carbon monoxide, which is a powerful reducing agent, rises through the charge, it chemically reduces the iron oxide - Fe2O3 + 3CO = 2Fe + 3CO2
This reaction takes place in the upper part of the furnace, where the temperature is too low to melt the Iron formed. The iron therefore, remains as a spongy mass until it moves down into the lower part of the furnace, where it melts are runs down over the white-hot coke, dissolving carbon, sulphur, mangaese, phosphorus and silicon as it goes. Apart from carbon, which is absorbed from the coke, these elements are also present due to the reduction of their compounds in the gangue.
At the same time as this reduction is taking place, the gangue, which is composed of mainly silica, combines with lime (formed by decomposition of limestone) to produce a slag -
CaCO3 - CaO + CO2
2CaO + SiO2 == 2CaO. SiO2 (calcium silicate slag)
Thus acid silica is neutralished by the basic lime. Limestone also removes sulphur.
According to the type of plant, the molten iron may be used in one or more of the following ways :- (1) Cast in pig bed, (2) Cast in pig-casting machine, (3) Transferred in hot metal ladles direct to an adjacent steel making process.
While the iron is in the furnace it picks up 3 to 4% carbon and a small amount of sulphur from the coke. The silicon, manganese and phosphorus as well as most of the sulphur in the pig iron are derived from the ore. The high amount of carbon makes the pig iron very hard and brittle and unsuitable for making any useful article. Pig iron is therefore, refined and remelted to produce other varieties of iron and steel.