CORROSION FATIGUE - Corrosion fatigue involves the reduction in the fatigue strength of a metal. This is due to the combined action of fatigue and corrosion. When a metal is subjected to cycle stress, the number of cycles required to cause failures at a given stress will be reduced in a corrosive environment below that in the absence of the environment. Basically, the corrosion provides better stress raisers by attacking already existing surface flaws such as cracks or by forming intergranular cracks where none existed earlier.
The stages in the development of fatigue cracks are as follows:
a) The formation of slip bands leading to intrusion or extrusion of material;

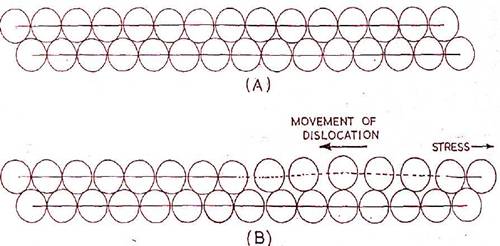
The Propagation of a "Dislocation" During Slip.
b) The nucleation of an embryo crack approximately 10um long ;
c) Extension of the embryo crack along favourable directions.
d)
Macroscopic (0.1 to 1 mm) crack propagation in a direction
perpendicular to the maximum principal stress, leading to
failure-
Examples of corrosion fatigue may be considered as taking
place in one of three different categories.
a)Active: freely corroding such as carbon steel in sea water.
b) Immune: In which the metal is protected either cathodically or with a coating.
c) Passive: In which the metal is protected by a corrosion generated surface film, usually an oxide.
FRETTING AND SELECTIVE CORROSION - Fretting corrosion takes the form of local surface discolouration and deep pits. Another type of localized corrosion is selective dissolution. The most common example is the dezincification of brass occuring in soft water service.
As the name implies, selective leaching is the net removal of one element from an alloy and is thus often referred to as deailoying or demetallificstion. The whole of an exposed surface may be attacked leaving the overall geometry unchanged, yet the removal of a large proportion of one of the alloying elements leaves a porous material with virtually no mechanical strength. Sometimes the effect is very localised, in which case perforation may occur.
The prime cause of dealloying is the galvanic effect between the different elements which compose the alloy, although other factors such as differential aeration and temperature are also important.
Components designed for use in both sea and fresh water have failed by this form of corrosion in such applications as condensers, valves, taps, pipe works as well as screws, nuts and bolts due to loss of either zinc, nickel, aluminium and tin from copper alloys. -
If the selective leaching proceeds over the whole surface it is termed uniform or "layer" dezincification. If corrosion in a local area proceeds inwardly it is called plug type. Selective dissolution of iron from cast irons is known as "Graphitization",
CREVICE
CORROSION -
Crevice corrosion occurs in cracks or crevices formed between mating
surfaces of metal assemblies and takes the form of pitting or etched
patches. Thus it is a localized form of corrosion. Both surfaces may
be of the same metal or of dissimilar metals or one surface may be a
non-metal. It can
also occur under scale and surface deposits and
under loose fitting washers and gaskets. Crevice corrosion is
believed to initiate as a result of differential aeration cell.
Crevice corrosion occurs only at film-protected metals such as
aluminium, magnesium, stainless steel and titanium. Poultice
corrosion is a special case of crevice corrosion, due to differential
aeration and usually takes the form of pitting when an absorptive
material such as paper, wood, asbestos, sacking, cloth etc is in
contact with metal surface that becomes wetted periodically. It
generally occurs with more anodic metals such as magnesium, zinc and
aluminium and common cathodic 'activators' are mercury and copper
ions in solution.
Thus crevice corrosion mainly takes place in oxide-passivated alloys by aggressive
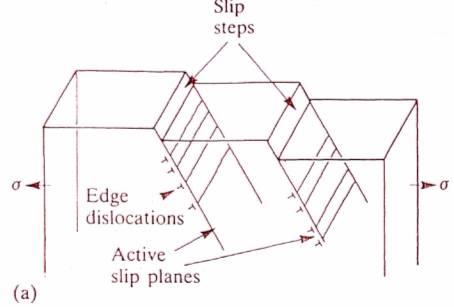
Metal
(b)
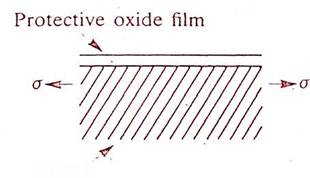
(The role of slip steps in environment-sensitive cracking.
(a) The formation of slip steps on the surface of a metal by movement of dislocations along active slip planes under the action of tensile stress.
(b) A slip step on the surface of a passivated metal creates an active site for the initiation of a stress concentrating pit.)
ions such as chloride in crevice or other shielded areas of a metal surface. Attack: on similar circumstances upon non-passivated metals is called differential aeration. The attack which occurs because part of a metal surface is in a shielded or restricted environment, compared to the rest of the metal which is exposed to a large volume of electrolyte.
MECHANISM- Crevice corrosion attack upon oxide-passivated alloys by aggressive ions such as chloride in crevices or other shielded areas of a metal surface is sometime termed as concentration cell corrosion. Attack in similar circumstances upon non-passivated metals is termed differential aeration corrosion.
Differential aeration corrosion on non-passivated metal occurs when a single piece of metal is exposed to electrolyte of varying concentration of oxygen. Crevices or cracks on metal surface having less air flow (oxygen concentration is less) act as anodes and the plane surfaces with more air (oxygen) act as cathodes. Like other concentration cells, the areas of low oxygen concentration become quickly corroded. Thus corrosion is greatest where there is least oxygen.
The attack by chloride ion upon a passivated metal surface due to crevice corrosion takes the following course -
i) Corrosion occurs slowly over the whole of the exposed metal surface both inside and outside the crevice with the electrolyte having uniform composition under normal anode & cathode processes. Under such condition, the generation of positive metal ions is counterbalanced electro statically by the creation of negative hydroxyl ions.
ii) The consumption of dissolved oxygen results in the diffusion of more oxygen from those electrolyte surfaces which are exposed to the atmosphere. Oxygen is more readily replaced at metal surfaces in the bulk electrolyte than at those within the crevice. Within the crevice, the lack of oxygen impedes the cathodic process and generation of negative hydroxyl ions is diminished within the confined space.
iii) The production of excess positives ions in the gap causes negative ions from the bulk electrolyte to diffuse into the crevice to maintain a minimum potential energy situation. In the presence of chlorides, it is likely that complex ions are formed between chloride, metal ions and water molecules. Hydrolysis takes place giving the corrosion product and hydrogen ions which reduce the pH. The simplified equation is M+ + H2O = MOH + H+
iv) The increase of hydrogen ion concentration accelerates the metal dissolution process and the metal within the crevice corrodes rapidly while that outside is cathodically protected.
6. PITTING - Pitting is a localized corrosion through the electrochemical reaction. It occurs when protective films generally continue and then break down in some environment producing cell action. The point of film breakage becomes an anode while the surrounding unbroken film acts as a cathode, effectively creating a small galvanic cell and producing pits. Corrosion products may form caps over pits cavities. While the shape of pits varies widely, usually, these are saucer-shaped, conical or hemispherical. Only a small amount of metal is corroded but perforations or hole that are developed can lead to costly repair of expensive equipment.
Pitting generally occurs on a metal surface where there is:
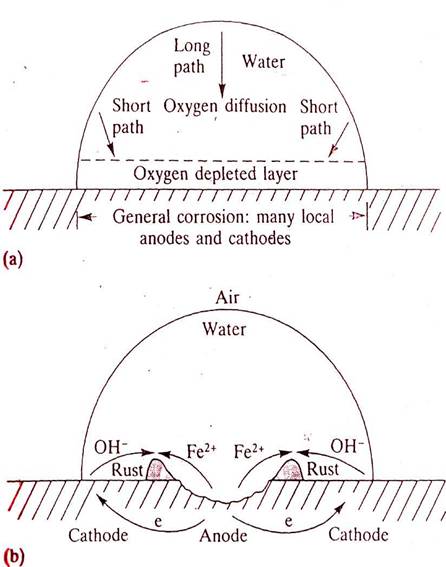
The mechanism of pitting because of differential-aeration beneath a water droplet.
(a)
General corrosion over the whole of the wetted metal surface depletes
the
oxygen levels in the adjacent electrolyte.
(b)
Longer diffusion path for oxygen to reach the central area makes this
the
anode. Metal dissolution occurs in the centre of the droplet
and reaction of metal
ions with hydroxyl ions formed at the edge
generates a ring of rust around the
corrosion pit.
(c) a surface scratch or other mechanically induced break in an Otherwise protective film
(d) An emerging dislocation or slip step caused by applied or residua! Tensile stresses
(e) A compositional heterogeneity such as an inclusion, segregate or precipitate.
MECHANISM
- This
corrosion mechanism is likely to occur when dissolved oxygen is
present in the electrolyte. The classic description of pit formation
beneath a drop of water on an iron surface is shown. The consumption
of oxygen by the normal cathode reaction in neutral solution causes
an oxygen concentration gradient within the electrolyte. Obviously,
the wetted area adjacent to the air/ electrolyte interface receives
more oxygen by diffusion than the area at the centre of the drop
which is at a greater distance from the oxygen supply.
This
concentration gradient anodic ally polarizes the central
region which actively dissolves : Fe
-> Fe2+ + 2e-
At the large Cathodic areas (scale protected plate) the electrons flow from the anode and are intercepted by the oxygen atoms present in the atmosphere, and in the presence of the water drop, they form hydroxyl ions -
H2O + 1\2O2 + 2e- -> 2(OH)-
As a result, the hydroxyl ions in the cathodic region diffuse inwards and the Fe2+ ions, being very small, migrate or diffuse outward, causing deposition of insoluble corrosion product Ferrous hydroxide Fe(OH)2 or brown rust. This further retards the diffusion of oxygen; accelarates the anodic process in the centre of the drop and then causes the reaction to be autocatalytic. In consequence corrosion proceeds as long as there is an available supply of fresh oxygen.
EROSION CORROSION/IMPINGEMENT CORROSION -
Erosion Corrosion refers to the acceleration of corrosion by simultaneous abrasion by the turbulent flow of gases or liquids. High velocity, a sudden change in the bore diameter or direction of a pipe, a badly fitting gasket or joint which introduces a discontinuity in the otherwise smooth metal surface, or presence of a deposit which may disturb laminar flow can cause impingement corrosion. Corrosion is initiated by the breakdown of a protective film at the place of impingement and its subsequent inability to repair itself under existing conditions.
Erosion action removes protective films from localised areas in the metal surface, and thereby contributes the formation of differential cells at such areas and to localised pitting at the anodic points of the cells. This type of corrosion is most common in condenser tubes, piping, agitator and containers in which streams of liquid energe from a pipe and strike a wall of the container.
FRETTING - A small relative movement between two metallic parts in close contact, can cause a form of mechanical wear, termed fretting. It is sometimes found on the backs of shell bearings and is due to slight movement of the bearing shell in its housing. Propeller shafts with a shrunk on liner and taper fitted propeller are sometimes prove to fretting troubles. The action occurs under the outboard end of the liner and under the forward end of the propeller hub. Fretting can occur in any area where there is a chafing action. The wearing action produces, in turn, wear particles which act as abrasives such as the chrome compounds from stainless steel.
FRETTING CORROSION - Normally, when not in use, steel is protected against corrosion to some degree by an oxide film. If this film is removed corrosion can be rapid as fresh metal is exposed to attack. Steels are especially prone to this form of attack as they react rapidly with moisture and oxygen. Fretting is a particular form of steel corrosion or corrosive wear, especially parts subject to vibration. This is characterised by the formation of very finely divided red ferrous oxide (Fe2O3). These fine particles are highly abrasive and can cause, excessive) wear despite the limited movement. The wear particles themselves, tend to further oxidise. A good example of fretting corrosion can be observed at the inner face of a roller bearing due to an unsatisfactory fit between the shaft and the race.
BRINELLING - It is a form of fretting in ball races of ball bearings caused by vibration of an otherwise stationery race. A grdual indentation results in the race. The name is taken from the Brinell hardness.
Ball bearings in stationery equipment are prone to this type of attack (sometimes termed "false brinelling"). When subject to vibration from an external source, even under unloaded or highly loaded conditions, and in the presence of a lubricant, the balls hammer the races locally, finally breaking the hardened surface to form small pit holes. The exposed metal then oxidises rapidly and corrode.
Greases containing mild load carrying additives have been found to overcome this problem.
INTERGRANULAR CORROSION- Intergranular corrosion is a form of localised surface attack in which a narrow path is corroded out preferentially along the grain boundaries of a metal because of the presence of precipitates in these regions. The driving force is a difference in corresion potential that develop between a thin grain boundary zone and the bulk of the immediately adjacent grains.
The precipitates may be intermetallics or compounds formed between metals and non-metallic elements. They can be either anodic, cathodic or neutral to the base metal.
Examples of anodic constituents are Mg5Alg, MgZn2 in aluminium alloys and Fe4N in iron alloys. Examples of cathodic constituents are FeAI3 and CuAI2 in aluminium alloys and Fe3C in iron alloys. If the precipitate is anodic to the denuded zone, it corrodes preferentially and if it is cathodic, the adjacent denuded zone is corroded. Intergranular corrosion occurs most commonly in aluminium, copper and 1 8/8 stainless steel.
In principle, there are three ways of reducing susceptibility of a metal such as a 304 stainless steel to intergranular corrosion - (i) use a low carbon steel i.e. less than 0.03% so that carbides are not stable, (ii) apply post-weld heat treatment to dissolve the precipitate, (iii) add titanium or niobium to form carbides preferentially.
MICROBIOLOGICAL CORROSION - Corrosion caused by the metabolic activity of various mircro-organisms is called microbiological corrosion. The more important micro-organisms responsible for most corrosion are (a) sulphate reducing bacteria, (b) sulphur bacteria, (c) iron and manganese bacteria and (d) micro-organisms capable of forming micro-bioligical films.
Metal pipes and structures buried in the damp soil containing sulphates and organic matter are attacked by the sulphate reducing bacteria. The corrosion product formed by such-an attack is iron sulphide instead of rust. Bacterial attack is fast and localised.
TIN OXIDE CORROSION IN BEARINGS -
The Metal :- Tin is a soft, malleable and ductile metal and its melting point is 232°C. Tin base white metal bearing has a typical composition of 89.3% tin; 8.9% antimony and 1.8% copper. In some cases, a small percentage of lead is incorporated to improve machinability and conformability. The addition of 1.0 to 1.5% cadmium raises the tensile strength and compressive strength.
Bearing Making :- The antimony in tin-base white metal forms an intermetallic compound SbSn. This compound forms cubic crystals called cuboids, which are hard and of low friction properties. These SbSn crystals are of lower specific gravity and they tend to float to the surface of the melt at higher temperature. However, the presence of copper, forms a network of needle-shaped crystals of Cu6Sn5 which precipitate from the melt before the cuboids of SbSn begin to form. This network traps Individual cuboids as they develop and prevents their movement towards the surface, thus ensuring a uniform cast structure. Moreover, the compound Cu6Sn5 being hard, improves the general bearing properties.
Whitemetal hardening :- The surface of tin-rich matrix of the whitemetal reacts chemically with oxygen, producing extremely hard tin-oxides, mainly stannic oxide (SnO2). It takes the form of extremely hard surface layer, either grey or grey-black appearance, the surface of which may be rough or relatively smooth.
New tin-based white metal has a Brinell hardness of about 30 to 40, but tin-oxide hardened layer hardness can be as high as 230 or about twice that of mild steel. Depending on the time and severity of attack, which is extremely indiscriminate, the hardened scale or layer may be from 0.13 to 0.76 mm). Ths entice bearing surface may be attacked or it may be only local areas. The relatively large tin-antimony cuboids (SnSb) and the thin copper tin needles (Cu6Sn5) remain unchanged.
There are several odd features of tin oxide formation is that below the affected area, the remaing metal is unchanged. While this is a chemical attack, penetrating into the alloy surface, particularly in severe cases, this is accompanied by an upward growth due to the volume increasing by oxygen absorption, so that ultimately bearing clearances are reduced.
Due to the formation of hard stannic oxide, the bearing alloy loses all its conformability and embeddability properties. Under such condition, if hard foreign matter enters the bearing, then the journal could become severely scored, whereas the originally soft white metal could be undamaged.
A greater danger, particularly with thick surface oxide layers, is that while these are extremely hard with a very high melting point, they are extremely brittle and susceptible to fatigue failure. If flakes of these layers become detached, they can cause serious damage to the journals, also completely take up the oil clearance space, causing a complete machinery breakdown.
Whitemetal hardening due to tin-oxide formation can be identified by grey or grey-black appearance and by hardness test. The clearance in a journal bearing will reduce and the bearing will run hot and possible scoring marks on the journal pin due to rubbing with extremely hard tin-oxide.
To bring the bearing into service, the hard stannic oxide have to be scraped off till the soft whitemetal reappears and the bearing clearance adjusted.
CONTROL AND PREVENTION OF CORROSION : Important methods are :
1) Use of metals of high purity and special alloy addition.
2) Modification of corrosive environment.
3) Application of inhibitor.
4) Cathodic protection.
5) Use of protective coatings.
6) Application of careful design principle.
METALS AND ALLOYS - The corrosion resistance of a given metal may be improved by increasing its purity. For example, high purity aluminium is subject to pitting corrosion to a lesser extent than normal casting alloys, and high purity copper does .not corrode once the surface film forms after initial exposure to the atmosphere. Impurities in metals are one of the main reasons why heterogeneity decreases the corrosion resistance of metals. However, it is possible to exhibit a high resistance to particularly severe corrosive environments by the addition of suitable and required amount of alloying elements. The high corrosive resistance of metals certainly could not be attained by pure metals only.
MODIFICATION OF CORROSIVE ENVIROMENT - Dearetion, dehumidification and purification of atmosphere can modify corrosion environment. This is done by reducing the air moisture or by filtering out of solid particles and absorption of corrosion constituents, from the atmosphere to such an extent that the amount of water condensed on metal surface is neglible to cause corrosion.
INHIBITORS - An inhibitor is a substance which retards or slows down a chemical reaction. Corrosion inhibitors are commonly added in small amount to acids, cooling waters, steam, etc. Inhibitors are classified in a number of ways - on the basis of environment such as acid, neutral and alkali; according to the observable effects of the inhibitors on the corrosion reactions such as anodic, cathodic and mixed. Inhibitors that suppress the anodic reaction or metal dissolution are called anodic inhibitors, which are oxidizing substances such as chromates, phosphates, silicates, nitrates and hydroxides are used, for the protection of iron and steel. These inhibitors seal off the anodic regions by forming oxide films.
Most of the organic inhibitors tend to shield the cathodic areas and can be grouped as Cathodic inhibitors. Organic inhibitors are. amines, mercaptaus, heterocyclic nitrogen compounds, sulphides and heavy metal soaps.
CATHODIC PROTECTION - Reduction or elimination of corrosion by making the metal cathodic by means of impressed current or attachment to a sacrificial anode (usually magnesium, aluminium or zinc) is known as cathodic or galvanic protection. In essence, no local currents are allowed to flow and thereby corrosion is retarded.
5. PROTECTIVE COATINGS - Two classes of coatings are applied a) non-metallic coatings and b) metallic coatings. The most common among non-metallic coatings are paints. Coatings of plastics, rubber, bitumen or pitch are non-metallic coatings of organic nature. They offer protection to the metal by providing a mechanical barrier. Chemical coatings are those that are formed by a chemical reaction on the metal surface. Metallic coatins can be applied by various processes such a (1) electroplating, (2) cladding, (3) hot dipping, (4) high temp, diffusion, (5) metal spraying and (6) vacuum deposition.
6. MODIFICATION OF DESIGN - The general principles of design to prevent or minimise corrosion may briefly be listed as under :-
a)Visual inspection of all surfaces to enable periodic removal of foreign matters like dust, dirt, soot, oil etc.
b)Avoidance of cracks and crevices.
c)Selection of proper metal for less corrosion current to generate in desired environment.
d)Separation of dissimilar metals from each other.
e)Making the anodic metal large and replaceable.
f) Making of cathodiq. protection.
g)Minimising residual and applied stress.