LOW PRESSURE EVAPORATORS
A considerable amount of fresh water is consumed in a ship. The crew consumes on average about 70 litre/head/day and in a passenger ship consumption 'per capita' can be as high as 225 litre/day.
The main object of distillation is to produce water essentially free of salts. Potablewater should contain less than 500 mg/litre of suspended solids. Good quality boiler feed will contain less than 2.5 mg/litre. Sea water on the other hand has a total dissolved solids (TDS) content in the range of 30,000 - 42,000 mg/litre, depending on its origin but, for most cases, the TDS is taken as 32,000 mg/litre.
Low pressure evaporators for the production of water can be operated to greatest advantage with engine cooling water on motorships. The relatively low temperature jacket water entering at about 65°C and leaving at about 60°C will produce evaporation because vacuum conditions reduce the boiling temperature of sea water from 100°C to less than 45°C.
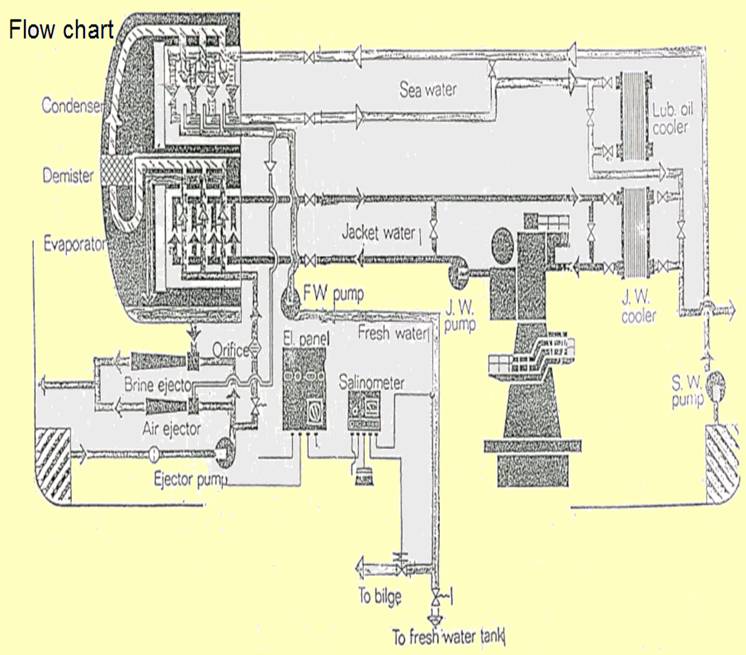
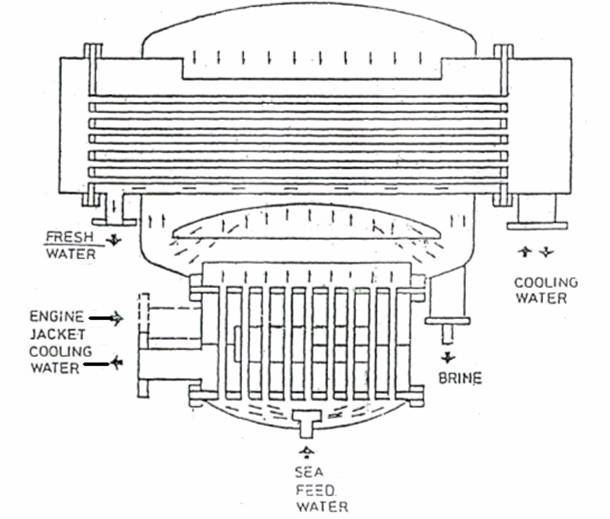
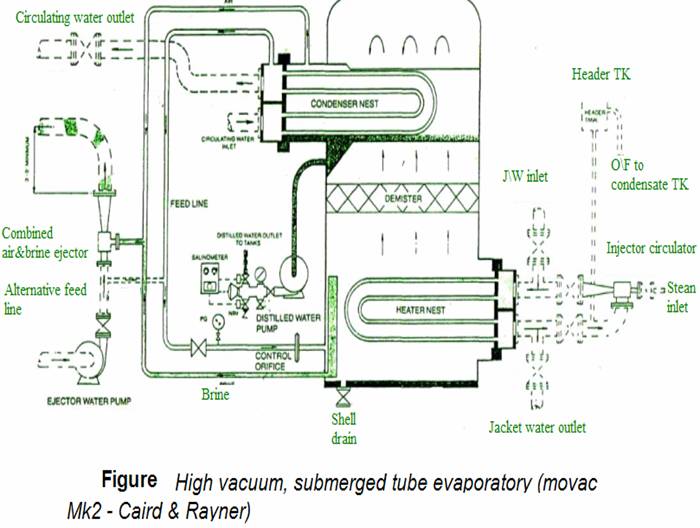
The single effect, high vacuum, submerged tube evaporator shown in Figure is supplied with diesel engine cooling water as the heating medium. Vapour evolved at a very rapid rate by boiling of the sea-water feed, tends to carry with it, small droplets of salt water which must be removed to avoid contamination of the product. The demister of knitted monel metal wire or polypropylene collects the salt-filled water droplets as they are carried through by the air. These coalesce forming drops large enough to fall back against the vapour flow.
Evaporation of part of the sea water leaves a brine the density of which must be controlled by continual removal through a brine ejector or pump. Air and other gases released by heating of the sea water, but which will not condense, are removed by the air ejector. The evaporator shown has a single combined ejector for extraction of both brine and air.
One of the gases liberated is CO2 from calcium bi carbonate. Loss of carbon dioxide from calcium bi-carbonate, leaves plain calcium carbonate which has poor solubility and a tendency to form soft, white scale. Other potential scale-forming salts are calcium sulphate and magnesium compounds.
Scale is not a major problem where submerged heating coils reach a temperature of only 60°C. This heat is too low for formation of magnesium scales and provided brine density is controlled, calcium sulphate will not cause problems. Continuous removal of the brine by the brine pump or ejector, limits density. Approximately half of the sea-water feed is converted into distilled water, the quantity of brine extracted is equivalent to the remainder of the feed delivered. The level of water in the evaporator is maintained constant by means of a brine weir, over which excess passes to the ejector.
The small quantity of soft calcium carbonate scale can be removed by periodic cleaning with a commercially available agent or the evaporator can be continually dosed with synthetic polymer to bind the scale-forming salts into a 'flocc' which mostly discharges with the brine. Use of continuous treatment will defer acid cleaning to make it an annual exercise. Without continuous treatment, cleaning may be necessary after perhaps two months. Steam heated evaporators with their higher heating surface temperature, benefit more from chemical dosing, because magnesium scales form when surfaces are at 80°C or more.